Poison Pill
Big Pharma stocks plopped into the dumper today like so many fecal pellets, thanks to the Vioxx debacle. Back in the day, that is, when I was a young sprout fresh out of my post-doc, Merck was the place to be if pharmaceutical research was your gig. How the mighty "America's Most Admired Company" has fallen. Roy Vagelos must be spinning in his...no wait, Dr. Vagelos is still alive. Well, he must be quite pained by what happened to his former company.
The idea behind the specific cyclooxygenase-2 (COX-2) inhibitors was a good one: target the enzyme suspected as being a prime mediator of inflammation, and leave its close relative, COX-1, which helps the stomach/gut lining stay happy and healthy, alone. In theory, this specific inhibition would spare the gut from ulceration and other nasty stuff which the less specific non-steroidal antiinflammatory drugs (NSAIDs) like ibuprofen can cause in some patients. There was a lot of nice biochemistry done on the COX-2 and COX-1 enzymes and their inhibitors. This research gave me that "ain't it cool?" tingly feeling, and also inspired me to think along the same mechanistic lines for a viral enzyme which was my research raison d’être for a few years. Several companies raced to the clinic with their COX-2 inhibitors, among them the aforementioned Merck.
New drugs are subjected to extensive clinical trials. Among the Vioxx trials were the VIGOR and APPROVe Phase III clinical studies. During the VIGOR clinical trial, in which Vioxx was compared to naproxen ("Alleve" is a brand of this OTC drug), there were indications that there was a four fold higher risk of heart attacks for the Vioxx treated patients enrolled in this clinical trial (0.4% of the Vioxx-treated vs 0.1% of the naproxen treated patients). Merck development scientists/clinicians believed that this was because of naproxen's cardioprotective effects although allegedly, there were rumblings via internal e-mails that Vioxx might be the cause. However, the APPROVe trial, which was designed to see if Vioxx would help prevent development of colon cancer, showed an increased risk of atherothrombotic ("clotting") events against control groups who were not taking naproxen. At that point, Merck could not dismiss the findings, and had to pull the drug.
The big question is...did Merck withhold negative results during the clinical trials? If this is the case, and there are indications that it is, Merck is going to have to pay the Litigation Piper. Withholding or suppressing clinical trial results is reprehensible. Gilmartin's (the former CEO) resignation speaks volumes.
Equally reprehensible were Merck's marketing tactics. Vioxx was originally targetted for a smaller group of patients, but was pushed aggressively by Merck for a much larger demographic. This demographic included patients with compromised cardiovascular health which made them more suseptible to the mechanism based toxicity of Vioxx. Also, keep in mind that not every physician is as skilled in pharmacology as he or she should be when whipping out those Rx pads. Some MD's may rely solely on pharma sales reps' information when they should also be keeping up with the medical literature. There's plenty of blame to go around.
Another problem was Merck's push to the FDA for "fast-tracking" Vioxx. This process is descibed more fully here. Its original intent was to move ahead new drugs directed toward unmet medical needs and for catasrophic diseases like AIDS or cancer. Whether Vioxx qualifies for this status is questionable to my bench monkey mind. Certainly, there were other drugs which, although not ideal, were taking care of inflammatory disease. Plus, diseases like osteoarthritis are chronic, and patients will be taking the drug for a long period of time, years perhaps, and safety is of the utmost concern. It would seem careful, extensive clinical trials to ensure safety would be in order, not fast tracking as appropriate for HIV and HCV antivirals or oncologics where adverse effects are more acceptable risks.
Hopefully, the whole fiasco will effect a sea change in pharma and in the FDA. In my opinion, one of the worst developments for the pharma industry was the event of direct to consumer (DTC) advertising for drugs. DTC marketing for Rx drugs is bad for the patient, bad for the physician and bad for the pharma company. There is a movement away from this. One big pharma has voluntarily imposed a 1 year moratorium on DTC ads for a year after a new drug is launched. Maybe others will follow suit.
Here is a truism: all drugs are poison - whether they help you or kill you is a matter of dosage. No drug is absolutely safe. The consumer must be well informed when it comes to medication, but given the level of scientific illiteracy in this country, I'm not optimistic that will ever happen. Elimination of DTC ads, more independent reviews of clinical trials (the FDA is a staggeringly bloated bureaucracy, so independent reviews will need to be conducted outside the agency), and better education of prescribing physicians (and not solely by pharma sales reps) would be positive steps.
That said, tort law reform is well overdue. There are drugs taken off the market which some patients desperately need. For the few percent, and tragically, this may be a fatal few percent, who experience adverse effects, there are many, the majority even, who do not, and for whom the drug is valuable. Then comes the hard decision: is the drug taken off the market because of exhaustive litigation? There are fewer and fewer obstetricians and anesthesiologists practicing due to soaring malpractice premiums which are driven by litigation.
Christ on celecoxib, I have a headache just thinking about all this. I think I'll go take...a pill.
___________________________________________
Addendum
Some answers to questions from "not up on this:"
What is Aleve? a NSAID?
Yes, Aleve is naproxen sodium, a non-steroidal antiinflammatory drug. It is a non-specific inhibitor of both cyclooxygenases, COX-1 and COX-2. These enzymes are both needed to make prostaglandins, an important class of biomolecules involved in all sorts of signaling processes in the body.
Does taking Advil also protect the heart for someone who can stomach it?
Where does Celebrex fit in this? Isn't it a NSAID too? Why or how is it diffrent than say Advil?
I'm going to address these questions by providing a bit of background on the balance of prostaglandins which activate platelets, and thus can cause them to stick together (clotting), and those which have antiplatelet properties (anti-clotting). For a straightforward explanation of platelets and what they do, check out this little article.
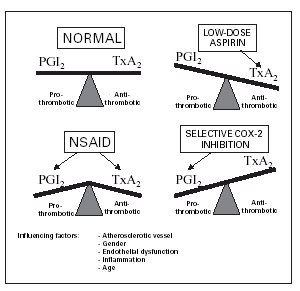
The prostaglandin which signals platelets to clump up and form a clot (this appropriately occurs when you get wounded) is called thromboxane A2 (TxA2). Platelets carry the COX-1 isoform which makes TxA2. Now COX-2, the other cyclooxygenase isoform, was long believed to be only upregulated as a response to biomolecules which induce inflammation. This inducible COX-2 was thought to be present only in cells which mediate inflammation, and thus was part of the impetus to seek COX-2 inhibitors to treat inflammatory diseases. However, it turns out that COX-2 is present in many tissues, including the vascular endothelium (this is the inner layer of cells lining blood vessels and has direct contact with blood). One of its key products is prostacyclin, or PGI2. PGI2 acts to prevent platelet aggregation. So, TxA2 and PGI2 levels are normally balanced to keep platelets in homeostasis. This is illustrated in the figure above in the upper left hand corner, e.g. the "normal" balance.
Any drug which inhibits cyclooxygenase can alter this balance. Low dose aspirin selectively inhibits COX-1 which decreases the level of the pro-clotting TxA2, and thus achieves an antithrombotic (anticlotting) effect (upper right hand panel; low levels of TxA2 = antithrombotic). Thus, low dose aspirin is recommended to prevent clotting. Now NSAIDs like naproxen and ibuprofen inhibit both COX-1 and COX-2. Thus, the levels of proclotting TxA2 and and the anticlotting/antithrombotic PGI2 are decreased. Because naproxen and ibuprofen also reduce PGI2, their cardioprotective effect may not be as good as that of low dose aspirin. The selective COX-2 inhibitors like Vioxx and Celebrex (also a selective COX-2 inhibitor like Vioxx; Bextra is another drug in this class) only decrease the "good" anticlotting PGI2 while leaving TxA2 levels alone. This selects for a pro-thrombotic/proclotting state (bottom right panel), and thus may increase the risk of heart attacks due to blood clots.
Bottom line: low dose aspirin appears to be the best bet for decreasing the risk of inappropriate clot formation, and Advil and Aleve are less so. It's likely that the risk for clot formation is a class effect in the COX-2 inhibitors. However, other vascular modulators may contribute to increased risk of thrombosis, and underlying disease in the patients being treated with these drugs must be considered, e.g., someone with distinct cardiovascular risk factors is probably not a good candidate to take this specific class of drugs.
Figure taken from F. Krotz et al. (2005) "Selective COX-2 Inihibitors and Risk of Myocardial Infarction," Journal of Vascular Research, 42: 312-324.
________________________________________________________________
Doc Bushwell's Fun With Science Glossary
Isoform: Multiple molecular forms of a given protein (or iso enzymes or isozymes if they are enzymes). Isoforms can usually be separated by electrophoresis or some other separation technique. They exist because of multiple gene loci or multiple alleles (also called allelomorphs / allelozymes or allozymes) or subunit interaction or secondary changes - such as post-translational modification. Definition courtesy of Glossary of Terms Used in Molecular Genetics.
post-translational modification. After a protein has been synthesized via the process of translation, e.g. DNA => RNA is transcription then RNA => protein is translation, other enzymes can come along and add embellishments to the protein. These may include addition of sugar moieties (glycosylation) or phosphate groups (phosphorylation) among others. Definition courtesy of what resides in Doc Bushwell's memory banks.

5 Comments:
What is Alleve? a NSAID?
Does taking Advil also protect the heart for someone who can stomach it?
Where does Celebrex fit in this? Isn't it a NSAID too? Why or how is it diffrent than say Advil?
not up on this,
Good questions! I'll add an addendum to answer these in due time.
- Doc Bushwell
nice write up. thanks.
Suesquatch,
You're too kind, and thanks!
As per your request, I've added a little glossary.
Doc Bushwell
"You really need to write this stuff for a lay science publication."
What's this place, transected hepatic parenchyma?
You're right, people who can communicate clinical and bench science in folksy ways as well as tie together the competitng economic, physiologic and sociologic realities of modern medicine are desperately needed.
Post a Comment
<< Home